Life History & Behaviour
Feeding
Ophiuroids possess a simple digestive system (see 'Anatomy and Physiology' tab) with no anus, which renders them unsuitable for extracting nutrients from large amounts of sediment (Stohr et al. 2012). Ophiuroids have a large range of feeding strategies including scavenging, predation, suspension-feeding and deposit-feeding (Ambrose Jr 1993; Stohr et al. 2012).
Ophiactis savignyi has more than one feeding strategy. This species can feed on detritus (McGovern 2002). They can also be suspension feeders by using their tube feet (or podia) to collect small particles of food from the water and move them towards their mouth (Ruppert et al. 2004; McGovern 2002). Lastly, O. savignyi can be a deposit feeder as it can filter food from the water and can also be situated at the excurrent pores of sponges to collect particles of food (Emson & Wilkie 1984; McGovern 2002).
Reproduction
Ophiactis savignyi can reproduce both sexually and asexually. This species is dioecious, meaning that the male and female reproductive organs are found in separate individuals (McGovern 2002). During sexual reproduction, the sperm is broadcast into the water and it is also presumed that eggs are broadcast, as brooding embryos have not been reported (McGovern 2002). The resulting larvae of sexual reproduction travel through the water column where they feed on plankton before they settle and metamorphose into juveniles (Emson & Wilkie 1984; Roy & Sponer 2002) O. savignyi are fissiparous brittle stars, meaning that they can split asexually into more than one individual (Emson & Wilkie 1984). This species reproduces asexually by disc fission, where the animal divides across the central disc, creating two individual brittle stars, each with three arms (McGovern 2002). The two individuals will then each regenerate three arms and the missing half of the central disc to form a whole brittle star (McGovern 2002).
Locomotion
Unlike asteroids, which use their tube feet for locomotion, ophiuroids rarely use their tube feet (or podia) for movement (Ruppert et al. 2004; Santos et al. 2005). Instead, ophiuroids use their arms for locomotion. While moving, ophiuroids hold their central disc above the substrate while the arms pull the animal forward (Ruppert et al. 2004). Usually, one or two of the arms pull the animal along while another one or two drag behind (Ruppert et al. 2004). The remaining arms make rowing movements to propel the animal forward in jerky movements (Ruppert et al. 2004). The spines on the arms are used for traction (Ruppert et al. 2004). This form of movement allows the animal to move in any direction, as there is no arm preference (Ruppert et al. 2004). The use of the arms for movement in ophiuroids also enables them to right themselves when they are upside down (see video below). Ophiuroids also have the ability to coil their arms around objects such as rocks to aid in their movement (Ruppert et al. 2004).
Respiration
Ophiuroid respiration occurs through the tube feet and bursae, which are invaginations of the oral surface of the central disc (Ruppert et al. 2004). There are ten bursae on each individual and they are blind sacs that open at slits on each side of the arm bases on the oral surface (Ruppert et al. 2004). Water enters through the slits due to the cilia on the bursae creating a current and it passes through the bursa and leaves via the slit at the end near the mouth (Ruppert et al. 2004).
Behavioural Study
INTRODUCTION
Ophiactis savignyi are benthic brittle stars that live in and under coral rubble on coral reef crests as well as in seagrass and mangrove habitats. The coral reef crest can be a harsh environment as it is exposed during low tide and sunlight is strong. As such, O. savignyi can be cryptic as it requires shelter from these environmental conditions.
The aim of this experiment is to determine whether O. savignyi display circadian rhythms and whether their behaviour is influenced by diurnal and tidal cycles. It is hypothesised that O. savignyi will spend more time hidden under coral rubble during daylight and will spend more time exposed while it is dark. It is also hypothesised that O. savignyi will spend more time out of the water during low tide and more time in the water during high tide as a result of a circadian rhythm.
METHODS
This study was undertaken in a laboratory during September 2013 on Heron Island, Australia (23.4420º S, 151.9140º E). Ophiactis savignyi (n=6) were collected from inside large coral boulders that had been collected on the reef crest at the north east point of Heron Island. The live specimens were placed in small plastic petri dishes with lids and their water was changed three times daily. Small pieces of coral rubble were also placed in each petri dish. O. savignyi were divided into two treatment groups, with one treatment group (Group 1) on a normal day/night cycle (n=3) while the other group (Group 2) was always subjected to darkness (n=3). The group on the day/night cycle was left uncovered between 6am and 6pm (daylight hours) and was covered by a styrofoam box between 6pm and 6am (daylight hours). A styrofoam box covered the second treatment group at all times. Observations for each individual (Group 1: 1, 2 and 3; Group 2: 4, 5 and 6) on whether the brittle stars were in the water or out, and hidden or exposed were made every three hours over a 48 hour period.

Image 1: Experimental set-up during the day. Plates on left are on day/night cycle and box covers plates in dark treatment.
RESULTS
The tidal cycle (Figure 1) or the time of day did not appear to influence the behaviour of O. savignyi in this study. It also appears that O. savingyi do not follow a circadian rhythm with regards to when they are hidden or exposed and in or out of water. However, the results did show a clear difference in behaviour between the two treatment groups, with Group 2 spending most of their time in the water (Figure 11, 12 & 13) and hidden (Figure 5, 6 & 7) compared to Group 1 which spent their time seemingly randomly both in and out of water (Figure 2, 3 & 4) and hidden and exposed (Figure 8, 9 & 10).
Figure 1: Tidal cycle at Heron Island over study period (26-28 September 2013).
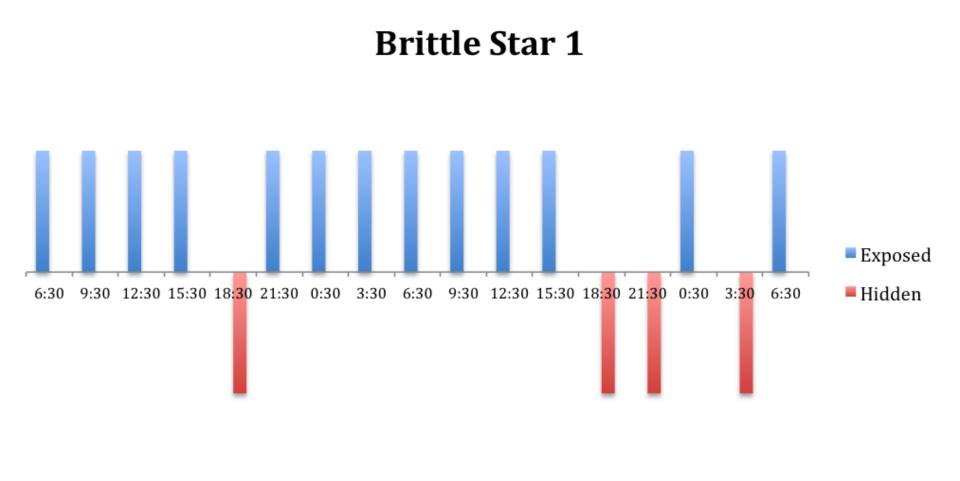
Figure 2: Behavioural observations (exposed or hidden) over time of O. savignyi
(No. 1) on day/night cycle.
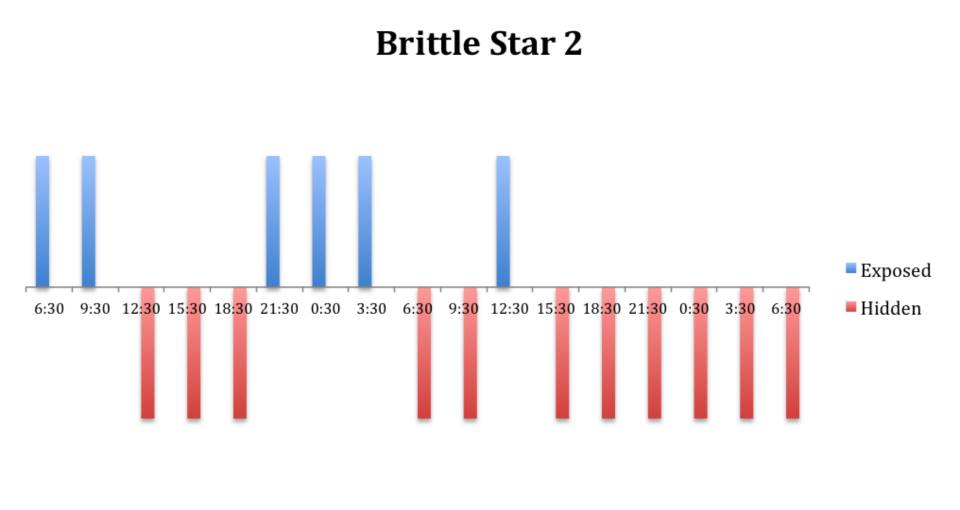
Figure 3: Behavioural observations (exposed or hidden) over time of O. savignyi
(No. 2) on day/night cycle.
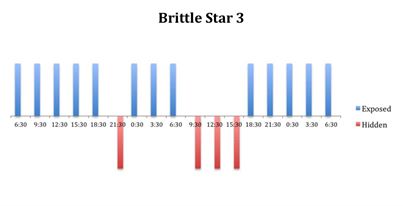
Figure 4: Behavioural observations (exposed or hidden) over time of O. savignyi
(No. 3) on day/night cycle.
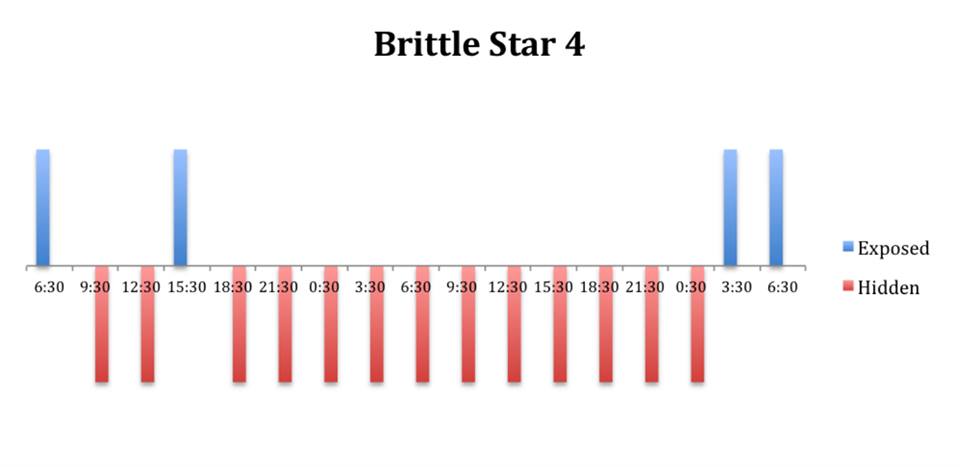
Figure 5: Behavioural observations (exposed or hidden) over time of O. savignyi
(No. 4) under constant dark conditions.
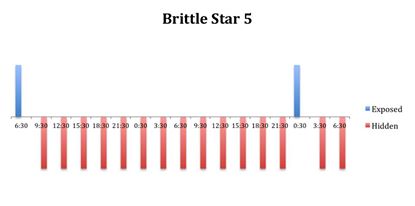
Figure 6: Behavioural observations (exposed or hidden) over time of O. savignyi
(No. 5) under constant dark conditions.
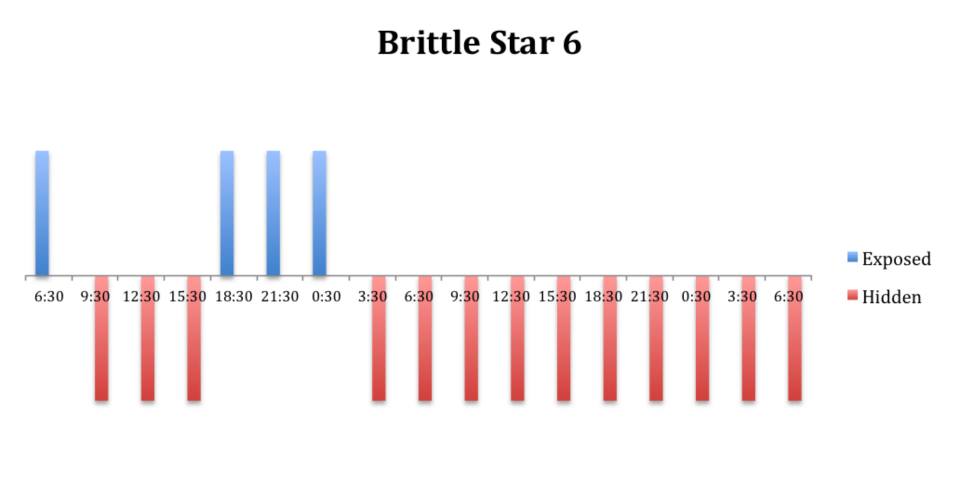
Figure 7: Behavioural observations (exposed or hidden) over time of O. savignyi
(No. 6) under constant dark conditions.
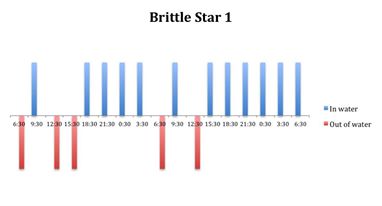
Figure 8: Behavioural observations (in water or out of water) over time of
O. savignyi (No. 1) on day/night cycle.
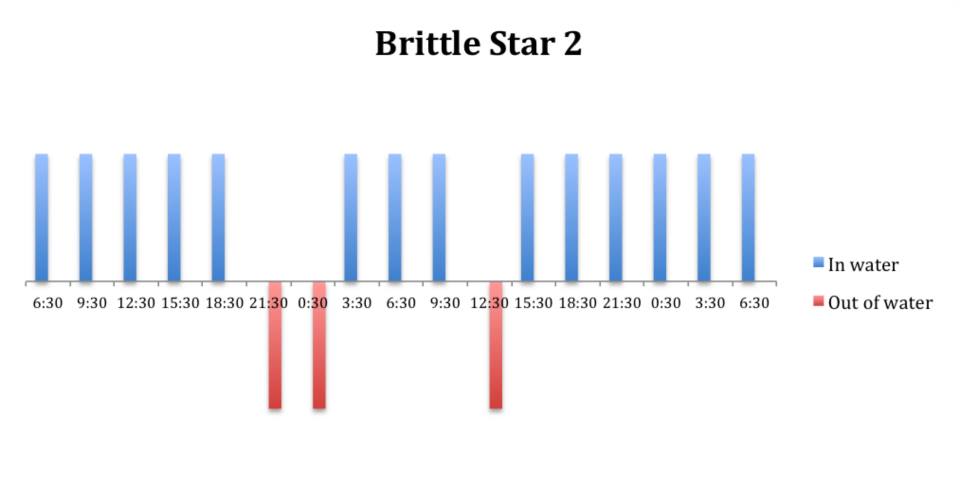
Figure 9: Behavioural observations (in water or out of water) over time of
O. savignyi (No. 2) on day/night cycle.
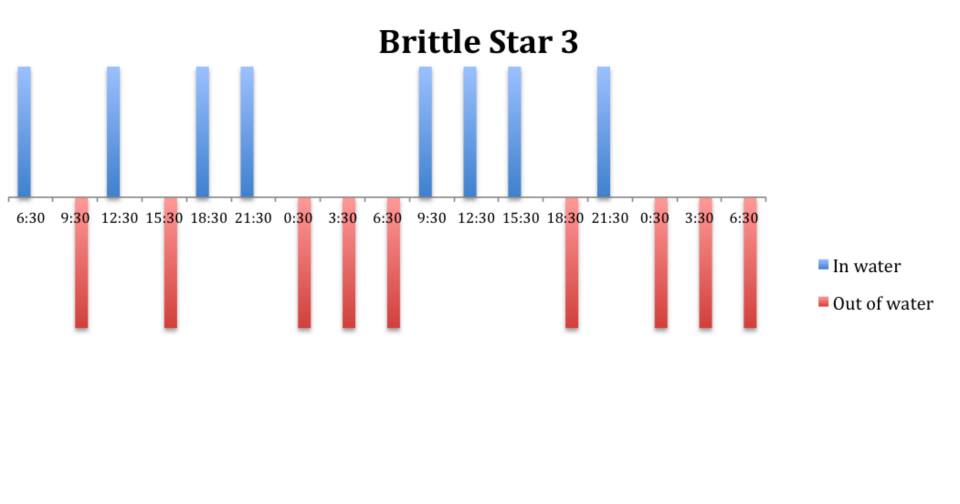
Figure 10: Behavioural observations (in water or out of water) over time of
O. savignyi (No. 3) on day/night cycle.
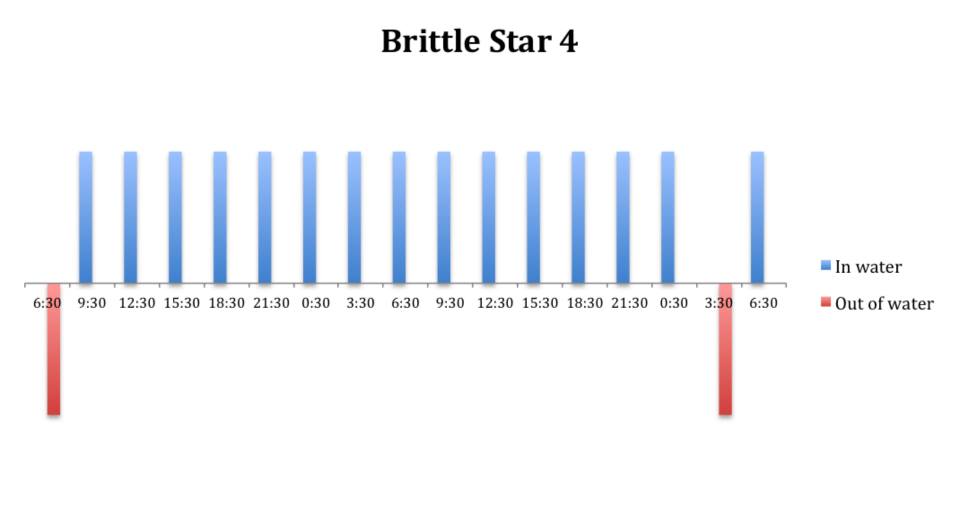
Figure 11: Behavioural observations (in water or out of water) over time of
O. savignyi (No. 4) under constant dark conditions.
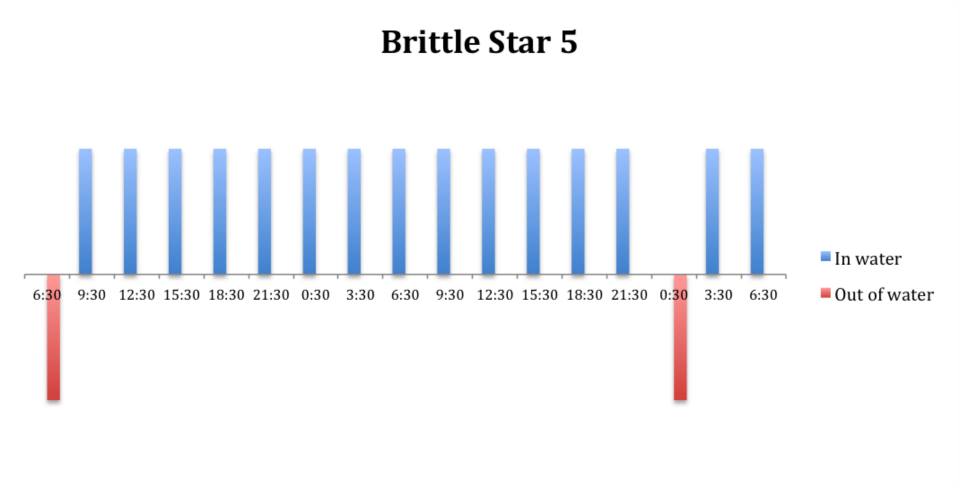
Figure 12: Behavioural observations (in water or out of water) over time of
O. savignyi (No. 5) under constant dark conditions.
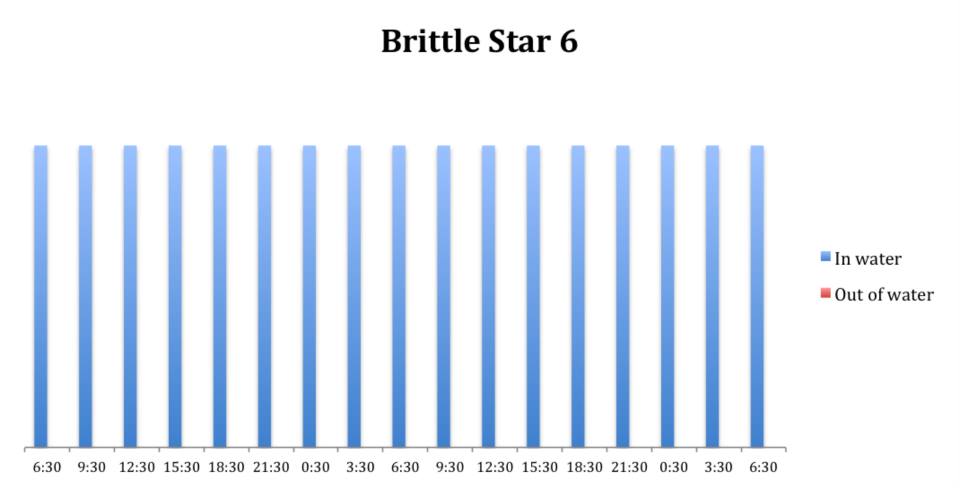
Figure 13: Behavioural observations (in water or out of water) over time of
O. savignyi (No. 6) under constant dark conditions.
|